ΔH 319 kJ. Pages 2 This preview shows.

Chem 101 Using Standard Enthalpies Of Formation And Standard Enthalpy Change Youtube
This is because enthalpy is a state function and we define Δi of an element to be zero when it is in its standard state.

. The following equation can be used to calculate the standard enthalpy of reaction. Using the table of standard formation enthalpies that youll find under the ALEKS Data tab calculate the reaction enthalpy of this reaction under standard conditions. Use standard enthalpies of formation to calculate the standard change in enthalpy for the meltin g of ice.
Hfreaction Hf p - Hf r if the reaction is H₂O s ----. Hess Law 2 More - Using standard enthalpies of formation 1 Calculate. Find step-by-step Chemistry solutions and your answer to the following textbook question.
Standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements with all substances in their standard states. Use standard enthalpies of formation to calculate ΔHrxn for the following reaction. Calculate delta Hf for the Cl- ions.
In places where we have more than one mole we multiply by the number of moles as seen in the balanced chemical equations. Add your answer and earn points. Use standard enthalpies of formation to calculate the standard enthalpy of reaction for the following reaction.
Many portable gas heaters and grills use propane C3H8g. ΔH rxn ΔH f productsΔH f reactants Δ H r x n Δ H f products Δ H f reactants. 2 H_2S g 3 O_2 g 2 H_2O l 2 SO_2 g DeltaH circ_ rxn sashamanger4796 is waiting for your help.
The change in enthalpy for dry ice sublimation is 339 kJ. To calculate ΔH for a reaction ΔH rxn 0 we simply need to use Eqn. These are elements in their standard sate and in that case the enthalpy of formaton is always zero.
92 kJ mol 1. Balanced equation balanced equation. The Δ H f Delta H_fcirc Δ H f.
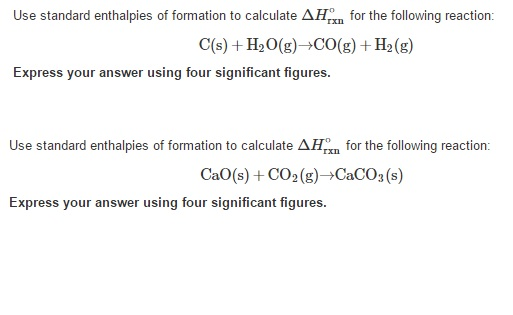
CaO s CO2 g CaCO3 s Express your answer using four significant figures. Hence what we would be considering are the standard enthalpies of formation of H2S H2O and SO2. Use standard enthalpies of formation to calculate Δ H rxn for the following reaction.
The zeros are the enthalpies for H 2 and Si. The standard enthalpies of formation are. ΔH reaction f f reactants where.
Weve got the study and writing resources you need for your assignmentsStart exploring. ΔH reaction Standard enthalpy change of formation expressed in kJ. C graphite 2H 2 g CH 4 g Δ H f -746 kJmol.
The standard enthalpy of formation formula for a reaction is as follows. The standard enthalpies of formation of ions in aqueous solutions are obtained by arbitrarily assigning a value of zero to H ionsthat is delta Hf Haq0 Afor this reaction. Given the standard enthalpies of formation ΔHf we can calculate the standard enthalpy change of sublimation ΔHs using the following expression.
The standard enthalpies of the molecules above are as follows. The enthalpy of reaction is calculated under standard conditions STP. Now when a compound is formed by combining any elements there is an enthalpy change which is the standard enthalpy of formation for that compound.
Use standard enthalpies of formation to calculate ΔrH for the following reaction. Course Title CHM 101. CO gH2O gH2 gCO2 g Express your answer using one decimal place.
CsH2OgCOgH2g Use standard enthalpies of formation to calculate ΔHrxn for the following reaction. SiCl 4 1 HClg. SO2g12O2gSO3g Use standard enthalpies of formation to calculate ΔHrxn for the following reaction.
Hf standard heat of formation can be use to claculate the std enthalpy change as follows. Answers - EXERCISE 3 - Hess Law 2 More - Using standard enthalpies of formation 1 Calculate the Hθ. C2H4 gH2 gC2H6 g Express your answer using one decimal place.
See the answer See the answer done loading. However we can use standard enthalpies of formationΔH f 0 as functional equivalents of a substances enthalpy. Its the change in enthalpy ΔH during the formation of one mole of the substance in its standard state pressure 10⁵ Pa 1 bar and temperature 25 C 29815 K from its pure elements f.
The standard enthalpy of formation of liquid pentane is -1468kJmol. ΔH products 2-20628 19029 -32227 kJmol. Use the following standard enthalpies of formation.
ΔH reactants 3332 1-2858 -1862 kJmol. To use standard enthalpies of formation to calculate the standard change in enthalpy for the melting of ice. Part B Use standard enthalpies of formation to calculate ΔrH for the following reaction.
NO g 903 kJmol and H₂O g -2418 kJmol. 2 NAOHsCaClLsCaOHs2 NaCls Round your answer to the nearest kJ. Emphasis mine In the above equation the elements are in their standard states and they are used to form one mole of ceMgO.
Cr₂O₃ s 3CO g 2Cr s 3CO₂ g ΔH rxn ΔH products - ΔH reactants ΔH products 20 3-393509 -1180527 kJmol. ΔH rxn -32227 - -1862 -13607 kJmol. Using enthalpies of formation calculate the quantity of heat produced when 160g of propane is completely combusted in air.
For example the standard enthalpy of formation for methane from carbon and hydrogen is -746 kJmol. L6 U2 Energetics Answers to exercise 3 - Hess Law 2doc -. 4NH₃ g 5O₂ g 4NO g 6H₂O g ΔH o reaction ΣΔH o f p ΣΔH o f r ΣΔH o f p 4molN O 903kJ 1molN O 6molH ₂O 2418kJ 1molH ₂O 3612 kJ 14508 kJ -10896 kJ.
Use standard enthalpies of formation to calculate Delta H circ _rxn for each reaction.
Solved Use Standard Enthalpies Of Formation To Calculate Chegg Com
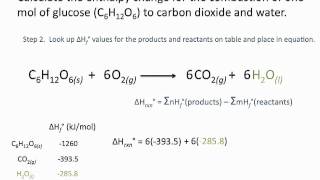
Enthalpies Of Formation Chemsitry Tutorial Youtube

Chemistry 101 Standard Enthalpies Of Formation And Reaction Youtube
0 Comments